The title of "world's smallest injectable pacemaker" is currently held by a device developed by scientists at Northwestern University, as of April 15, 2025. Here's a breakdown of this innovative technology:
Key Features of Northwestern University's Injectable Pacemaker:
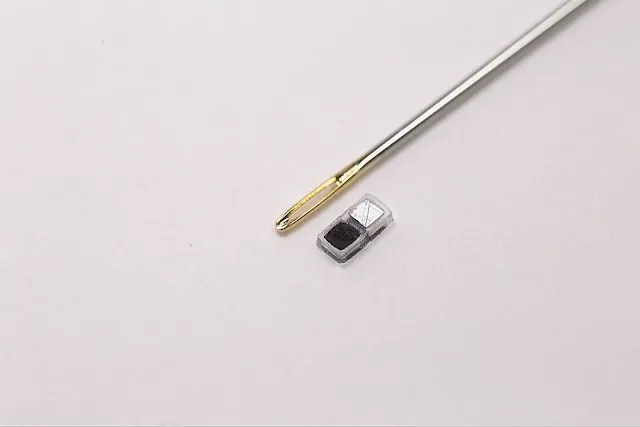
* Size: Smaller than a grain of rice (approximately 1.8 mm in width, 3.5 mm in length, and 1 mm in thickness).
* Injectable: Designed to be non-invasively injected into the body using a syringe, eliminating the need for traditional surgical implantation.
* Temporary Pacing: Primarily intended for patients who require temporary pacing, such as newborn babies with congenital heart defects after surgery or adults recovering from heart surgery.
* Wireless Control: Paired with a small, soft, flexible, and wearable device that is placed on the patient's chest. This wearable device detects irregular heartbeats and then automatically shines a light pulse (infrared wavelength) through the skin to activate the implanted pacemaker.
* Light-Activated: The pacemaker responds to these light pulses, which penetrate safely into the body, to regulate the heartbeat.
* Battery-Free: It operates using a galvanic cell, a simple type of battery that generates electricity from the body's own fluids. Two different metals within the pacemaker act as electrodes. When they come into contact with surrounding biofluids, a chemical reaction occurs, producing an electrical current to stimulate the heart.
* Dissolvable: Made from biocompatible materials that naturally dissolve into the body's biofluids once the temporary pacing is no longer needed, thus avoiding the need for a second surgery to remove it.
Potential Benefits:
* Minimally Invasive: Injection via syringe significantly reduces the invasiveness of pacemaker placement.
* Reduced Risk of Complications: Eliminates the risks associated with surgical incisions and lead wires used in traditional pacemakers.
* Specifically Suited for Newborns: Its small size makes it particularly beneficial for infants with congenital heart issues.
* No Need for Removal Surgery: The dissolvable nature avoids the need for a subsequent surgical procedure.
* Potential for Integration: Its tiny size could allow integration with other implantable devices like heart valve replacements.
Current Status:
* The device has been successfully tested in mice, rats, pigs, dogs, and human heart tissue in the lab.
* Researchers estimate that human trials could potentially begin within the next two to three years.
Other Small Pacemakers:
It's worth noting that before this light-activated, dissolvable pacemaker, Medtronic's Micra Transcatheter Pacing System (TPS) was widely recognized as the world's smallest commercially available pacemaker.
* Micra Size: Approximately the size of a large vitamin capsule (about 25.9 mm in length and 6.7 mm in diameter, with a volume of 0.8 cc and a weight of 2.0 g).
* Leadless: Implanted directly into the right ventricle of the heart via a catheter inserted through a vein in the leg, eliminating the need for leads.
* Permanent Pacing: Designed for long-term bradycardia (slow heart rate) management in adults.
* Battery Life: Offers a battery life ranging from 8 to 13 years.
* MRI Conditional: Some Micra models are MRI SureScan™, meaning they are safe for MRI scans under specific conditions.
While the Medtronic Micra is significantly larger than the newly developed injectable pacemaker, it is an established and widely used leadless pacing technology. The new Northwestern University device represents a groundbreaking advancement towards even smaller and less invasive cardiac pacing, particularly for temporary needs and potentially for vulnerable populations like newborns.
Another leadless pacemaker that was developed was the Nanostim (by St. Jude Medical, now Abbott). It was also a small, leadless device implanted directly into the heart. However, it was recalled in 2016 due to battery malfunctions. This highlights the ongoing evolution and challenges in the field of leadless pacing technology.




Comments
Post a Comment